Nutrients Can Be Concentrated From Dilute Solutions by
Saturated solutions can be. This is a high ratio therefore the solution of HCl HCl is concentrated.
희박하다 Images Stock Photos Vectors Shutterstock
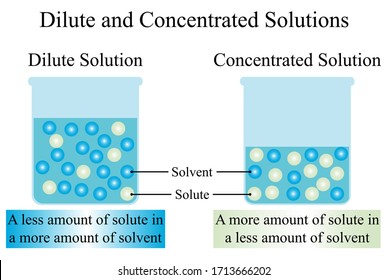
A concentrated solution is one in which there is a large amount of substance present in a mixture.
. Nutrients can be concentrated from dilute solutions by active transport and group translocation Which of the following is MOST effective against resistant endospores. Adilute bconcentrated ceither dilute or concentrated dneither dilute or conce Get the answers you need now. For example consider solution of sparingly soluble salt in water.
Look dilution is directly related to molarity now ur question is that how can we dilute solution into half of its concentration it means that if we have 01 molar solution then we have to prepare it 005 molar and we want to dilute it in 100cm3 of. Albumin tests are used to diagnose kidney disease and. The supply of K is more constant with a lower level in the starter solution and a more concentrated refill solution.
Hence the solution is dilute. A solution can be prepared in different concentration according to the amount of solute dissolved in it. The amount of fertilizer that should be used to make the concentrate is typically dictated by the injection ratio.
A solution can be both was asked on May 31 2017. A dilute solution is a solution that has very little solute in the solvent. Nutrients can be concentrated from dilute solutions by A.
WSFs are meant to be dissolved or solubilized in water before use. Also the amount of solute present is very small compared with the amount of solvent. Active transport and group translocation.
What is the only type of mixture that has particles that dissolve and are transparent. 2 Osmosis involves the passage of solvent from a dilute solution or pure solvent into a more concentrated solution. Saturated and unsaturated d.
A solution can be dilute or concentrated. 100 100 dm3 dm 3 of HCl HCl is added to 10 10 dm3 dm 3 of water. Dilute and concentrated b.
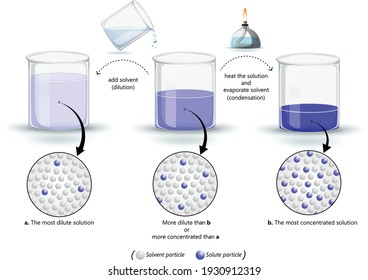
Making concentrated solutions for further dilution. The terms dilute solution and concentrated solutions are vague as they lack quantitative preciseness. Particles from a mixture mixing together but then eventually settling down to the bottom.
Saturated and dilute c. A solution can be both saturated and dilute. However if the urine is highly concentrated it will contain more albumin than normal human serum albumin HSA.
Concentrated urine can be measured using an albumin test in which a sample of urine is sent to a laboratory to determine how much albumin it contains. 001 001 g g of MgOH2 Mg OH 2 is added to 1 000 1 000 dm3 dm 3 of water. You can make a solution more concentrated by adding __________.
Concentration is termed in a qualitative way through which the use of adjective such as dilute. If the urine is dilute then it will contain little or no albumin. The terms concentrated and dilute are used to describe solutions.
Fertilizer injectors are used by most growers to apply water-soluble fertilizers to plants. View the answer now. Hence it is a saturated solution.
Hawthornes WSF products such as General Hydroponics FloraPro are fertilizer blends produced from high quality technical grade fertilizer salts in dry powder form. The main difference between the dilute solution and concentrated solution is that dilute solution contains less solute and the concentrated solution contains more solute. Supersaturated and saturated e.
Some nutrients such as calcium and magnesium may be mixed into the growing medium prior to planting but most of the nutrients are applied after planting using water-soluble fertilizers. Solutions that contain relativity large amount of solute are called concentrated and relatively small amount of solute are called dilute Dilutio is simply adding water to. A liquid with a high solute concentration is called a concentrated solution.
This is a low ratio therefore the solution of MgOH2 Mg OH 2 is dilute. These devices inject a small quantity of concentrated fertilizer solution stock solution into the. The ___ is the thing being dissolved.
A common practice growers employ is to prepare a concentrated fertilizer and use fertilizer injectors or proportioners to dilute the fertilizer into the irrigation system. 1 To find the molarity of a solution both solution volume and the number of moles of solute must be known. Ammonium nitrate NH4NO3 can be added to the pH control solution if necessary to obtain even higher levels of N in the plants but ammonium reduces the uptake of other cations so it should only be used if necessary.
Water Soluble Fertilizers WSF are a great source of nutrients to use for growing indoor hydroponic crops. No more solute can be dissolved in such a solution. All these combinations are opposites of each other and a solution can not be both at the same time therefore the answer is e.

Dilute Images Stock Photos Vectors Shutterstock

Plantboost Plant Nutrient Solution In 2022 Plant Nutrients Solutions Plants

Concentrated Vs Dilute Solution What Is A Concentrated Solution Video Lesson Transcript Study Com
Comments
Post a Comment